Abstract
This post explores current advances, where new additive manufacturing methods and strategies could revolutionize drug and therapeutic delivery, and the impetus driving the idealized future of on-demand engineering of personalized medicine leading to parallel innovation in drug manufacturing and delivery in the healthcare and pharmaceutical industry.
1. Introduction
Ever had the need to pop several pills at different times throughout a day? It is a pain.
The need to deliver appropriate therapeutic for an individual has been a long-standing challenge for the medical industry. Today medicinal drugs typically involve appropriate dosing of an active compound with respect to a patient, or specific route of administration of the drug (oral, intravenous, nasal, epidermal, etc.), specifically controlled release formulation [1] or specialized drug nanoparticles [2].
The problem with Drug Delivery Systems (DDS) is that it is a challenge to administer the appropriate quantity of therapeutic agents in humans at the right time. Administering drug dosage typically involves the bioengineering of optimal drug pharmacokinetics, efficacy, management and understanding of absorption and metabolism of the drug by controlling the effect and target sites, release rates and mechanism within a human body [3].
Today, almost all pharmaceutical companies use compression force to mold-form a pill, but this limitation in long-standing traditional manufacturing processes typically restricts the ingredient variability and manufacturing flexibility of the type and dosage of medication that can be produced [4], and employing new DDS formulation or chemistry into an antiquated manufacturing line is often economically unfeasible.
The advantages of 3D-printing have brought about tremendous new capabilities in other industries and are well documented in terms of advantages and limitations [5] and medical professionals have begun to evaluate the potential of 3D-printing drugs to capitalize on this new approach for better drug delivery [6, 7].
2. Current Landscape
A major problem in healthcare today is the challenge of dosing. It is unfeasible for pharmaceuticals to manufacture numerous pill sizes and dose concentrations for every single patient where drug doses are often dependent on patient weight and frequency of administration depending on the pathology.
For example, drug X comes in 1mg or 10mg tablets, but what happens when the dosage for a child’s weight requires 7mg? A doctor could prescribe a dose of seven 1mg tablets or instruct the guardian or parent to break a 10mg tablet into half and take a half-tablet (5mg) and two 1mg tablets.
As you can imagine, amenability will not be good and the tendency to mistake the dosage could lead to an under- or over-dose of the medication. Both are undesirable. This is where 3D-printing has been explored extensively in personalized medicine (PM).
3D-printing drugs enables customization and modification of medication to fit individuals to reduce side effects (specific doses) or mitigate the need for multiple-pill regime (multi-ingredients).
PM generally consists of designing effective treatment formulation to meet the attributes and specific needs of each patient, with the objective of administering the right drug at the precise dose and appropriate time [8], regularly involving data-driven insights from metabolic and genotype testing of the patient [9].
3D-printing drugs enable caregivers to engineer the release profiles of a drug structurally and chemically without changing the active therapeutic ingredient, and numerous approaches have been studied [10, 11]. In 2015, the FDA approved the world’s first 3D-printed tablet Spritam (levetiracetam), to treat onset seizures and epilepsy [12, 13]. The structure allows the tablet to dissolve instantly within the patient’s mouth, a major characteristic desirable when doctors are prescribing the drug to children and patients with dysphagia.

Another immediate appeal of 3D-printing drugs is personalized dosing [14] to avoid medication-related toxicities associated with extremely potent drugs such as theophylline and prednisolone [4, 15], and side effects associated with unsuitable drug concentrations with regards to the patient’s mass [16, 17]. Today, patients must take multiple pills of the same type or break up pills to achieve the desired concentrations – a tedious task that is inconvenient and prone to errors, especially when a pill regime occurs several times a day.
Additional benefits that 3D-printing drugs bring are controllable drug release profiles that are achieved by engineering variable solubilities of printed polymeric materials [16]. By depositing soluble, insoluble or hygroscopic polymers to encapsulate the active pharmaceutical agent [18–23], or by structural engineering a break-away design [24], researchers were able to print a cough-expectorant guaifenesin with similar release profiles as the commercial tablet [25], and by varying the thinness of the capsule, two time-delayed doses could be released in one capsule [26].
Besides specific-release capsules enabled by 3D-printing, a new generation of therapeutics referred to as a “polypill” leverages on 3D-printing to produce physical structures in an encapsulation design that contains multiple therapeutic agents of different release profiles.
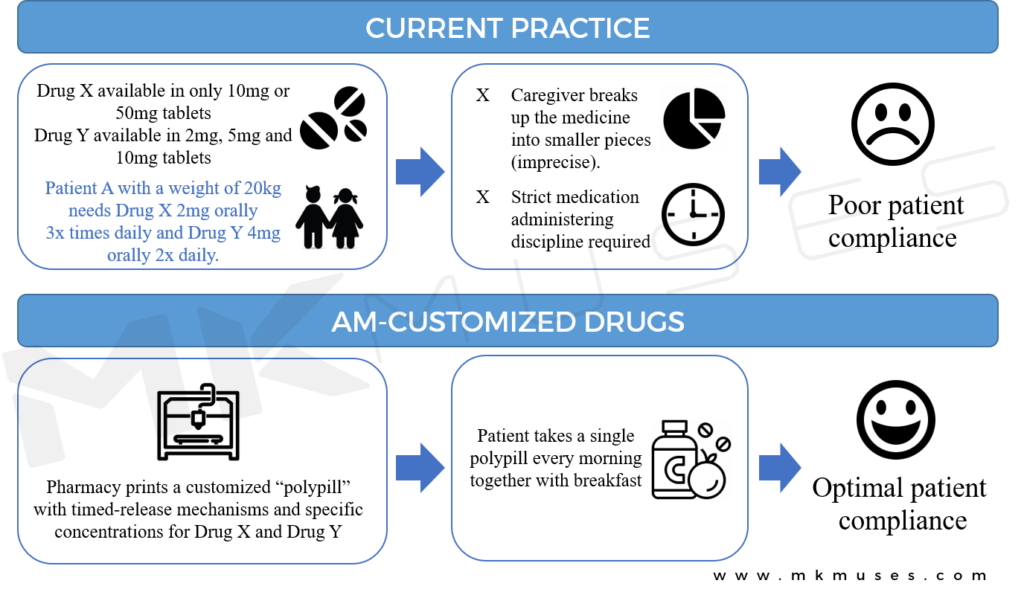
The capacity to embed several active pharmaceutical ingredients in a single pill that can be designed to dissolve to fit specific eluting profiles cannot be understated. 3D-printed polypills mean that profile-specific compartments can be printed in different sections of the pill to release the necessary dose at different times for improved patient outcomes.
Today, researchers have printed multiple different drugs into a single polypill [27–30], demonstrating the feasibility of 3D-printing as a driving platform for multi-drug therapy and an era of specific and personalized medication prescription.
Polypills that feature timed-delayed doses [26] and with multiple therapeutics in one capsule [27] and/or both characteristics [29] is particularly beneficial to juvenile and elderly patients in improving compliance to a medical regime as a single polypill replaces multiple pills, often an unpleasant activity when one has to take numerous pills several times a day [31, 32].

Today, complex structures of 3D-printed tablets with specific-release dosage and profiles have been extensively designed [11, 29, 33, 34] with the objective of improving patient compliance when patients suffer from conditions that require a cocktail of medications orally ingested at different times of the day.
In fact, it is possible for drugs to be administered only during disease emergent events, and 3D-printing is the enabler of an even newer generation of “reactive” or “adaptive” drugs.
3. New Opportunities
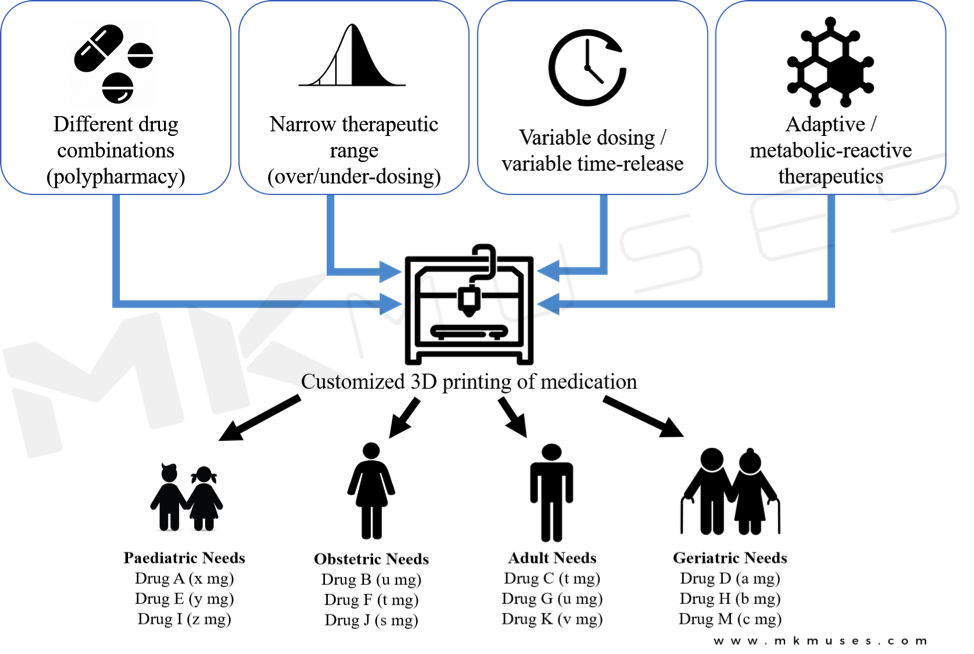
3D-printed drugs distinguish themselves from traditional manufacturing processes via structural engineering, on-demand fabrication of the medicine and precision dosing. Figure 4 lists a few advantages where 3d printed drugs will benefit different patient needs and requirements. However, today’s 3D-printed drugs simply involve the deposition of a therapeutic agent into a type of polymeric binding material. Is there possibly an even better way to deliver drugs as and when they are needed?
Recent advances in DDS include nanomedicine “smart” drugs with smart-targeting capabilities [35], where drug releases are triggered by stimuli and released on-demand instead of mere delayed release via polymer dissolving mechanisms [36]. Such extended-release smart nanoparticle-based drugs [2, 37–40] could be loaded into a 3D-printing system to enhance the capabilities of a polypill even further.
A smart polypill perhaps?
Patients under customized therapeutics displayed improved outcomes much quicker than patients who did not undergo pharmacogenetic testing [33]. Smart polypills could also benefit patients who are suffering from chronic but infrequent illnesses where the drugs are released when the smart nanoparticles are activated by targeted markers. In fact, there is already an emerging field in PM referred to as “theranostics” [41, 42], where specific targeted nanomedicine therapy is based on individual diagnostic tests [43].
The boundaries of smart adaptive drugs that react to the changing biochemistry of a patient are further challenged with the idea of “chemprinters” – the 3D printing of actual molecules. Researchers at the University of Glasgow first printed microfluidic channels [44], then progressed to the digitization of a multi-step process that eventually allowed them to print out molecules [45], watch a YouTube video of their process here.
In 2012, Dr. Lee Cronin delivered a very inspiring TED talk on his team’s vision of a 3D printer that can print out therapeutic molecules with a visionary future of printing your own medicine using chemical inks, watch his TED talk here.
The team’s vision is that once molecular chemistry can be printed, with a few universal “reagent inks”, therapeutic agents and medicinal chemistry are very possible in the near future [46].
Imagine being able to download the organic blueprint for a desired molecule and a futuristic pharmaceutical printer that is able to print downloadable drugs on demand.
4. Challenges
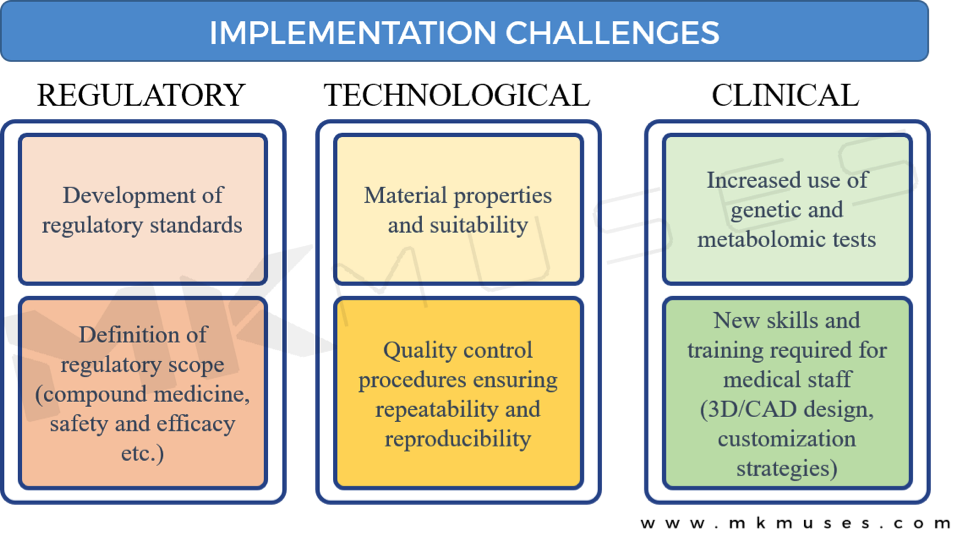
As with new and exciting technologies where 3D-printing is advancing the field of drug delivery, there are challenges that need to be addressed before 3D-printed drugs become mainstream [8].
Regulatory Challenges
When different therapeutic drugs are compounded in a pharmaceutical setting in a printed polypill, the varying practices and types of equipment used will present a challenge in quality assurance and compliance.
How will the FDA qualify and access the varied amalgamations of medicines in a single tablet that has not ever been studied in a clinical trial? Although the FDA encourages the development of 3D-printing technologies and is currently updating its regulations to represent the nascent field of 3D-printed drugs to protect public health, these questions remain. In 2015, the FDA’s Centre for Drug Evaluation and Research’s Emerging Technology Team issued a draft guidance providing recommendations to pharmaceutical companies on new medical technologies such as 3D printing and 3D-printed drugs [47], and the promise is that FDA is engaged to address and eliminate potential barriers to the adoption of 3D-printed drugs [48].
Technological Challenges
Quality and safety will no doubt be a priority, and a recurring challenge is the reliability and purity of reagents and print cartridges and/or materials available to democratize 3D-printed drugs. Standards in pharmaceutical manufacturing facilities are high and meet international standards, so the key matter is how the same standards translate in a smaller pharmacy environment. A major concern is that contaminated or defective materials may yield a faulty drug product that may pose an even greater threat than the 3D printer.
Moreover, the limited availability of suitable bio-materials and understanding of the compatibility requirements of different drugs and printing conditions will pose a challenge to healthcare professionals [34].
Clinical Challenges
The various stakeholders will need to consider the potential medical liability implications. For example, is a digital design or schematic for a polypill sufficient grounds for responsibility in the event of an adverse incident or a malpractice suit? Or is it the manufacturer of the 3D printer, the biomedical engineer who printed the pill or the drug companies who provided the excipient or active ingredients?
Whilst there has been no published litigation involving 3D-printed therapeutics yet and therefore no precedent, the question here is that the involvement and level of liability of the various parties are not immediately clear and each participant across the manufacturing spectrum could be liable for legal consequences. Pharmaceutical companies developing and producing 3D-printed drugs will need to develop a strategy protecting and licensing digital drug designs legally.
5. Conclusion
Challenges notwithstanding, there is little doubt that 3D-printing promises incredible advantages over existing drug-manufacturing technologies plus prescription practices and promises to improve the quality of life.
The advantages of 3D-printied drugs discussed in this paper outweigh the challenges mentioned. The ability to optimize dosage and reduce multi-drug regimens provides both long-term and immediate wellbeing results for chronic patients with a range of ailments.
3D-printing drugs are poised to drive the adoption of PM, and the benefit it brings to reducing medication-related side effects and compliance cannot be understated [8]. Patients are far likelier to comply with a reduced pill regime, and increased adherence leads to better disease management and better health outcomes [28, 29].
I think that the potential of adaptive/smart printable drugs on demand is a future yet to be explored and this newest advancement in medicine and continued innovation involving 3D-printed therapeutics is an exciting field that will prove that the individual patient will be the greatest ultimate beneficiary.
6. References
1. Santos, H., et al., Mesoporous materials as controlled drug delivery formulations. Journal of drug delivery science and technology, 2011. 21(2): p. 139-155.
2. Allen, T.M. and P.R. Cullis, Drug delivery systems: entering the mainstream. Science, 2004. 303(5665): p. 1818-1822.
3. Jain, K.K., Drug delivery systems. Vol. 2. 2008: Springer.
4. Norman, J., et al., A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Advanced drug delivery reviews, 2017. 108: p. 39-50.
5. Chua, C.K. and K.F. Leong, 3D printing and additive manufacturing. 2014: World Scientific.
6. Hsiao, W.-K., et al., 3D printing of oral drugs: a new reality or hype? 2018, Taylor & Francis.
7. Lamichhane, S., et al., Complex formulations, simple techniques: Can 3D printing technology be the Midas touch in pharmaceutical industry? Asian Journal of Pharmaceutical Sciences, 2019. 14(5): p. 465-479.
8. Trivedi, M., et al., Additive manufacturing of pharmaceuticals for precision medicine applications: A review of the promises and perils in implementation. Additive Manufacturing, 2018. 23: p. 319-328.
9. Aneesh, T., et al., Pharmacogenomics: the right drug to the right person. Journal of clinical medicine research, 2009. 1(4): p. 191.
10. Lepowsky, E. and S. Tasoglu, 3D printing for drug manufacturing: A perspective on the future of pharmaceuticals. Int J Bioprint, 2018. 4(1): p. 119.
11. Jamróz, W., et al., 3D printing in pharmaceutical and medical applications–recent achievements and challenges. Pharmaceutical research, 2018. 35(9): p. 176.
12. Fitzgerald, S., FDA Approves First 3D-printed epilepsy drug experts assess the benefits and caveats. Neurology Today, 2015. 15(18): p. 26-27.
13. Kumar, H., et al., Three-dimensional drugs: A new era in the pharmaceutical development. Indian journal of pharmacology, 2017. 49(6): p. 417.
14. Alomari, M., et al., Personalised dosing: printing a dose of one’s own medicine. International journal of pharmaceutics, 2015. 494(2): p. 568-577.
15. Sandler, N., et al., Inkjet printing of drug substances and use of porous substrates‐towards individualized dosing. Journal of pharmaceutical sciences, 2011. 100(8): p. 3386-3395.
16. Moulton, S.E. and G.G. Wallace, 3-dimensional (3D) fabricated polymer based drug delivery systems. Journal of Controlled Release, 2014. 193: p. 27-34.
17. Preis, M. and H. Öblom, 3D-printed drugs for children—are we ready yet? AAPS PharmSciTech, 2017. 18(2): p. 303-308.
18. Melocchi, A., et al., Hot-melt extruded filaments based on pharmaceutical grade polymers for 3D printing by fused deposition modeling. International journal of pharmaceutics, 2016. 509(1-2): p. 255-263.
19. Maroni, A., et al., Erodible drug delivery systems for time-controlled release into the gastrointestinal tract. Journal of Drug Delivery Science and Technology, 2016. 32: p. 229-235.
20. Melocchi, A., et al., 3D printing by fused deposition modeling (FDM) of a swellable/erodible capsular device for oral pulsatile release of drugs. Journal of Drug Delivery Science and Technology, 2015. 30: p. 360-367.
21. Zhang, J., et al., Hydroxypropyl methylcellulose-based controlled release dosage by melt extrusion and 3D printing: Structure and drug release correlation. Carbohydrate polymers, 2017. 177: p. 49-57.
22. Kempin, W., et al., Assessment of different polymers and drug loads for fused deposition modeling of drug loaded implants. European Journal of Pharmaceutics and Biopharmaceutics, 2017. 115: p. 84-93.
23. Trenfield, S.J., et al., 3D printing pharmaceuticals: drug development to frontline care. Trends in pharmacological sciences, 2018. 39(5): p. 440-451.
24. Arafat, B., et al., Tablet fragmentation without a disintegrant: A novel design approach for accelerating disintegration and drug release from 3D printed cellulosic tablets. European Journal of Pharmaceutical Sciences, 2018. 118: p. 191-199.
25. Khaled, S.A., et al., Desktop 3D printing of controlled release pharmaceutical bilayer tablets. International journal of pharmaceutics, 2014. 461(1-2): p. 105-111.
26. Maroni, A., et al., 3D printed multi-compartment capsular devices for two-pulse oral drug delivery. Journal of Controlled Release, 2017. 268: p. 10-18.
27. Robles-Martinez, P., et al., 3D printing of a multi-layered polypill containing six drugs using a novel stereolithographic method. Pharmaceutics, 2019. 11(6): p. 274.
28. Khaled, S.A., et al., 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. Journal of controlled release, 2015. 217: p. 308-314.
29. Khaled, S.A., et al., 3D printing of tablets containing multiple drugs with defined release profiles. International journal of pharmaceutics, 2015. 494(2): p. 643-650.
30. Park, K., 3D printing of 5-drug polypill. Journal of Controlled Release : Official Journal of the Controlled Release Society, 2015. 217:352.
31. Cargill, J.M., Medication compliance in elderly people: influencing variables and interventions. Journal of advanced nursing, 1992. 17(4): p. 422-426.
32. Tashkin, D.P., Multiple dose regimens: impact on compliance. Chest, 1995. 107(5): p. 176S-182S.
33. Okwuosa, T.C., et al., A lower temperature FDM 3D printing for the manufacture of patient-specific immediate release tablets. Pharmaceutical research, 2016. 33(11): p. 2704-2712.
34. Palo, M., et al., 3D printed drug delivery devices: perspectives and technical challenges. Expert review of medical devices, 2017. 14(9): p. 685-696.
35. Anderson, J.M. and S.W. Kim, Recent advances in drug delivery systems. 2012: Springer Science & Business Media.
36. Davoodi, P., et al., Drug delivery systems for programmed and on-demand release. Advanced drug delivery reviews, 2018. 132: p. 104-138.
37. Kalaydina, R.-V., et al., Recent advances in “smart” delivery systems for extended drug release in cancer therapy. International journal of nanomedicine, 2018. 13: p. 4727.
38. Muñoz-Juan, A., et al., Latest Advances in the Development of Eukaryotic Vaults as Targeted Drug Delivery Systems. Pharmaceutics, 2019. 11(7): p. 300.
39. Patra, J.K., et al., Nano based drug delivery systems: recent developments and future prospects. Journal of nanobiotechnology, 2018. 16(1): p. 71.
40. Sun, H., Y. Zhang, and Z. Zhong, Reduction-sensitive polymeric nanomedicines: an emerging multifunctional platform for targeted cancer therapy. Advanced drug delivery reviews, 2018. 132: p. 16-32.
41. Chen, G., et al., Upconversion nanoparticles: design, nanochemistry, and applications in theranostics. Chemical reviews, 2014. 114(10): p. 5161-5214.
42. Kelkar, S.S. and T.M. Reineke, Theranostics: combining imaging and therapy. Bioconjugate chemistry, 2011. 22(10): p. 1879-1903.
43. Lim, E.-K., et al., Nanomaterials for theranostics: recent advances and future challenges. Chemical reviews, 2015. 115(1): p. 327-394.
44. Kitson, P.J., et al., Configurable 3D-Printed millifluidic and microfluidic ‘lab on a chip’reactionware devices. Lab on a Chip, 2012. 12(18): p. 3267-3271.
45. Kitson, P.J., et al., Digitization of multistep organic synthesis in reactionware for on-demand pharmaceuticals. Science, 2018. 359(6373): p. 314-319.
46. Symes, M.D., et al., Integrated 3D-printed reactionware for chemical synthesis and analysis. Nature chemistry, 2012. 4(5): p. 349-354.
47. Coburn, J.C. and G.T. Grant, FDA regulatory pathways and technical considerations for the 3D printing of medical models and devices, in 3D Printing in Medicine. 2017, Springer. p. 97-111.
48. Di Prima, M., et al., Additively manufactured medical products–the FDA perspective. 3D printing in medicine, 2016. 2(1): p. 1-6.